Our Services
Clinical Trials
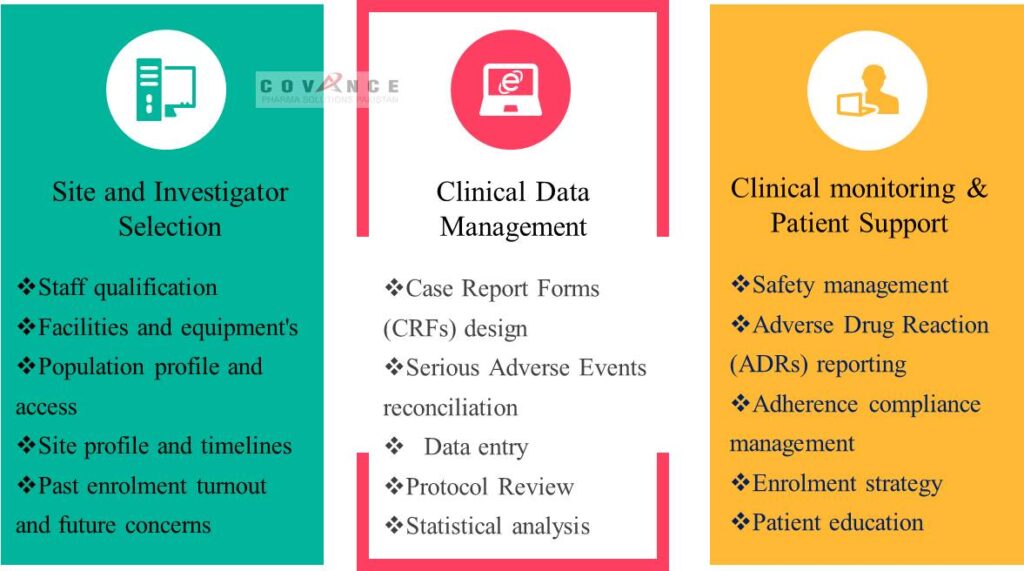
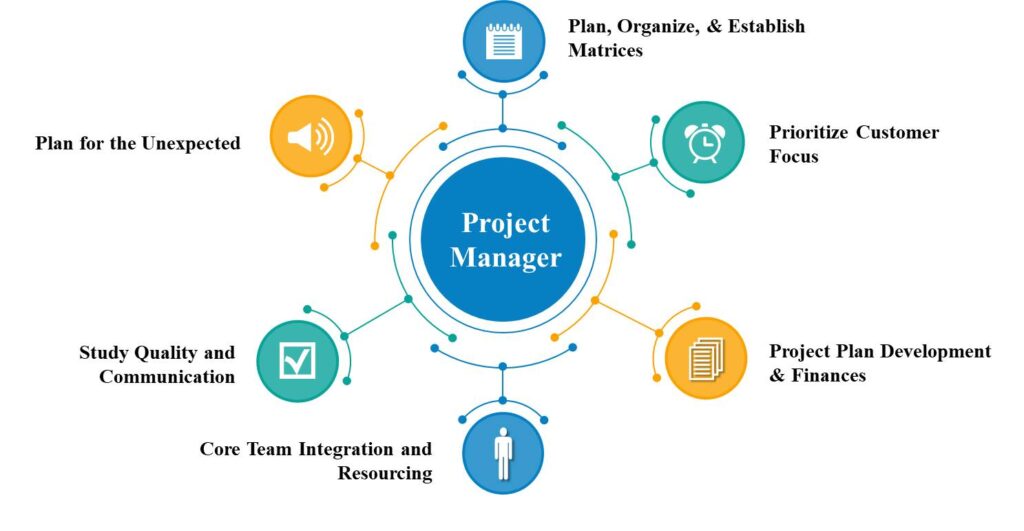
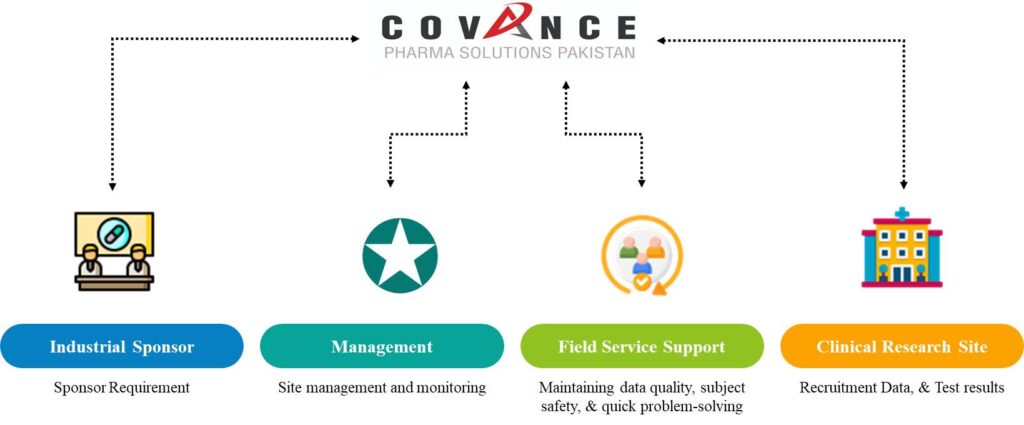
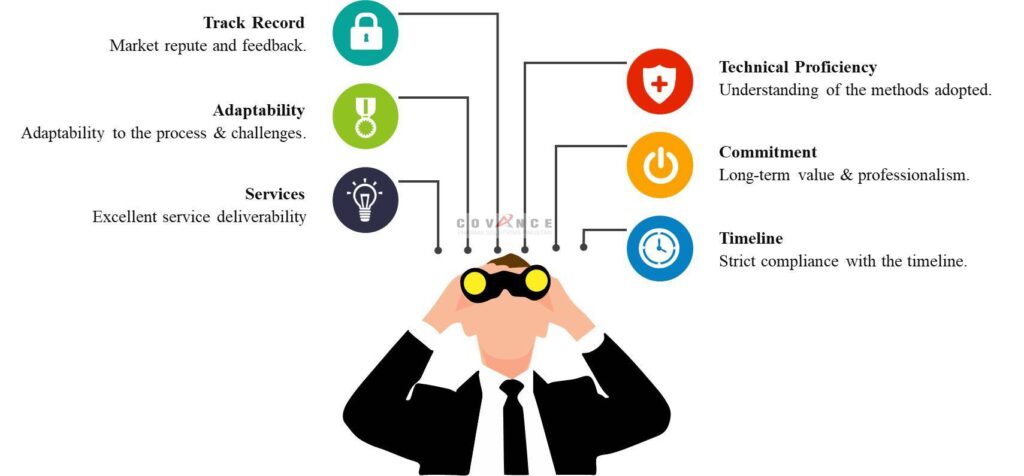
We provides comprehensive range of Clinical trial services in Pakistan based on Compliance, Optimism, Regulation & Ethics (CORE)

FAQ
General Questions
Drug Regulatory Authority of Pakistan (DRAP) is mandated to regulate the Clinical Trials and to implement internationally recognized standards as adopted by World Health Organization (WHO) and International Conference on Harmonization (ICH). The authority with approval of Federal Government, has notified the Bio-Study Rules 2017 for regulation of the clinical trials activities in the country.
The CSC is responsible to assess that rights, safety and well-being of trial participants are protected before the start of clinical trial. This committee reviews the protocols and monitor the compliance to Good Clinical Practices (GCP) and cause inspection of Clinical Trial Site and CROs.
National Bio-Ethics Committee is a notified body on bio-ethics working under the Ministry of National Health Services, Regulation and Coordination.

