Our Service
BA/BE Studies
BA/BE Assistance and Services
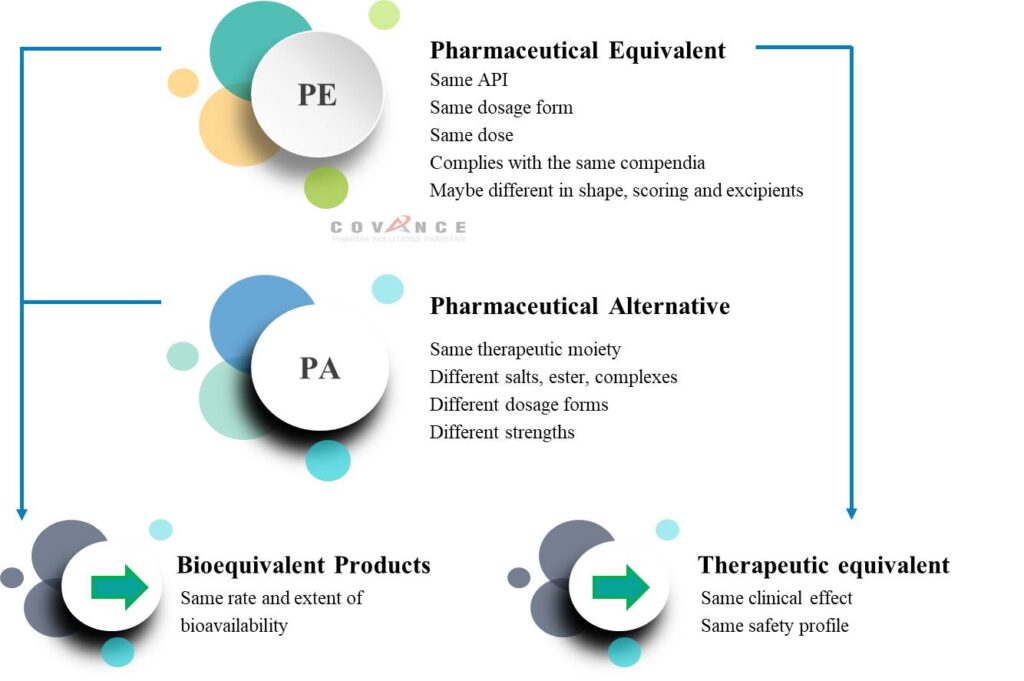
Two medicinal products containing the same active substance are considered bioequivalent if they are pharmaceutically equivalent or pharmaceutical alternatives and their bioavailabilities (rate and extent) after administration in the same molar dose lie within acceptable predefined limits. These limits are set to ensure comparable in vivo performance, i.e. similarity in terms of safety and efficacy.
Need for Bioavailability and Bioequivalence studies
– Universal approach about comparative bioavailability
These investigations are carried out in order to determine the quantitative nature of a particular product comparison. The performance of a modified release formulation in comparison to a conventional capsule or the pharmacokinetic parameters of an oral formulation in comparison to an intravenous dose are assessed using the absolute bioavailability of a new drug. It is primarily done for a generic product to compare a competitive formulation to a reference or standard drug. These similarities in comparative bioavailability studies point to a common experimental strategy.
– Comparative bioavailability studies of new drugs (NDA)
To ascertain the bioavailability and bioequivalence of the formulation in humans for safety and efficacy, comparative bioavailability studies are used. By comparing the pharmacokinetics parameters of an intravenous and oral administration of new drug formulations with the same dose, it is possible to learn more about the bioavailability of a given drug formulation.
– Comparative bioavailability of generic drugs (ANDA)
It is not necessary to carry out the entire batch of clinical trials required for the first product when a manufacturer seeks therapeutic equivalence in order to market a competitive generic product. If therapeutic equivalence has been established, the study must be conducted in accordance with the established study requirements and must be comparable to or equivalent to the earlier product or innovator. Therapeutically, this is thought to be equivalent to the innovative drug product.
– Testing under fasting conditions or fed conditions
The drug can also be tested under fed conditions to meet all requirements as per regulatory norms in bioequivalence studies if it does not produce the expected results under fasting conditions.·

